An acid is a substance with a pH lower than seven. Its name derives from the Latin word “acidus,” which means “sour.” An acid has a sour taste and sharp odor. It is also a base if it takes a proton from another substance. Water is an acid. The name of an acid is derived from the chemical formula H2O.
Acids are compounds, which donate protons and accept electrons. They react with metals to produce hydrogen gas. They react with bases to form salts. The more acidic is an alkaline solution and the strongest is water. Both acids and bases can be neutral and have an ionic strength of zero. An acid and a base are made up of the same chemical formula, but an acid is more reactive than a base.
A base is an acid that accepts electrons and donates protons. An acid is known as an Arrhenius acid if it reacts with aqueous solutions. In non-aqueous solvents, it is known as a Bronsted acid. It contains a hydrogen atom bonded to its chemical structure. Its energy is favorable even after losing H+. In addition, it can act as an electrolyte or a base.
A base is an acid with a pH lower than four. Its pH ranges from two to four, and a strong acid can be dangerous to your health. A strong base can cause burns or lead in your eye. It is corrosive to all types of materials, and should be kept away from children. It should not be mistaken for a neutral substance. A weak base is a substance that reacts with metals.
An acid is a substance that has more hydrogens than a base. It has a sour or tart taste and is a highly acidic substance. It reacts with metals to liberate hydrogen, while a base accepts hydrogen ions from the environment. An acid is neutralized with the use of a base. Once you have a clear indicator of the chemical compound, you can determine its pH level.
An acid changes the colour of indicators when it reacts with iron, while a base reacts with iron to create a salt. Both types of acids have the same function: to promote a chemical reaction. A base can be mineral or organic. An acid can be a base or a sulfate. Unlike a base, it can change the color of a substance. As long as it reacts with a metal, it is an acid.
When a chemical reacts with another substance, it can react with both acids and bases. An acid acts as a donor of protons while a base absorbs electrons. Basically, an acid is a solution of hydrogen and oxygen. A base is the opposite of an agent. The former reacts with metals to release hydrogen, while an acid absorbs protons from its surroundings. The latter reacts with water to produce a salt.
An acid reacts with water to give it a blue tint. An acid can accept an electron from a base. It also reacts with metals to release hydrogen. An acid can be a base. If a reaction is between an acid and a base, it is an example of a weak acid. A strong acid can be a neutralizing agent. A weak acid will react with a neutral substance.
An acid is a substance that contains hydrogen. Its colour is red. When the indicator is blue, it is an acid. It will react with iron to release hydrogen, while a base will react with a base to give it a neutral state. Both of these types of acids have different properties. A strong acid will turn a blue litmus paper red. A weaker acid will not react with a metal.
As the name suggests, acid is the substance that can give or accept hydrogen ions. A base, on the other hand, can take a proton. Its pH value is lower than seven. A weak acid has a higher pH than seven. An unreactive base is a strong acid. The last one is called a hydronium ion. These are both an example of acids. If an acid has an ion, it is an aqueous mixture of hydrogen and carbonates.























































































































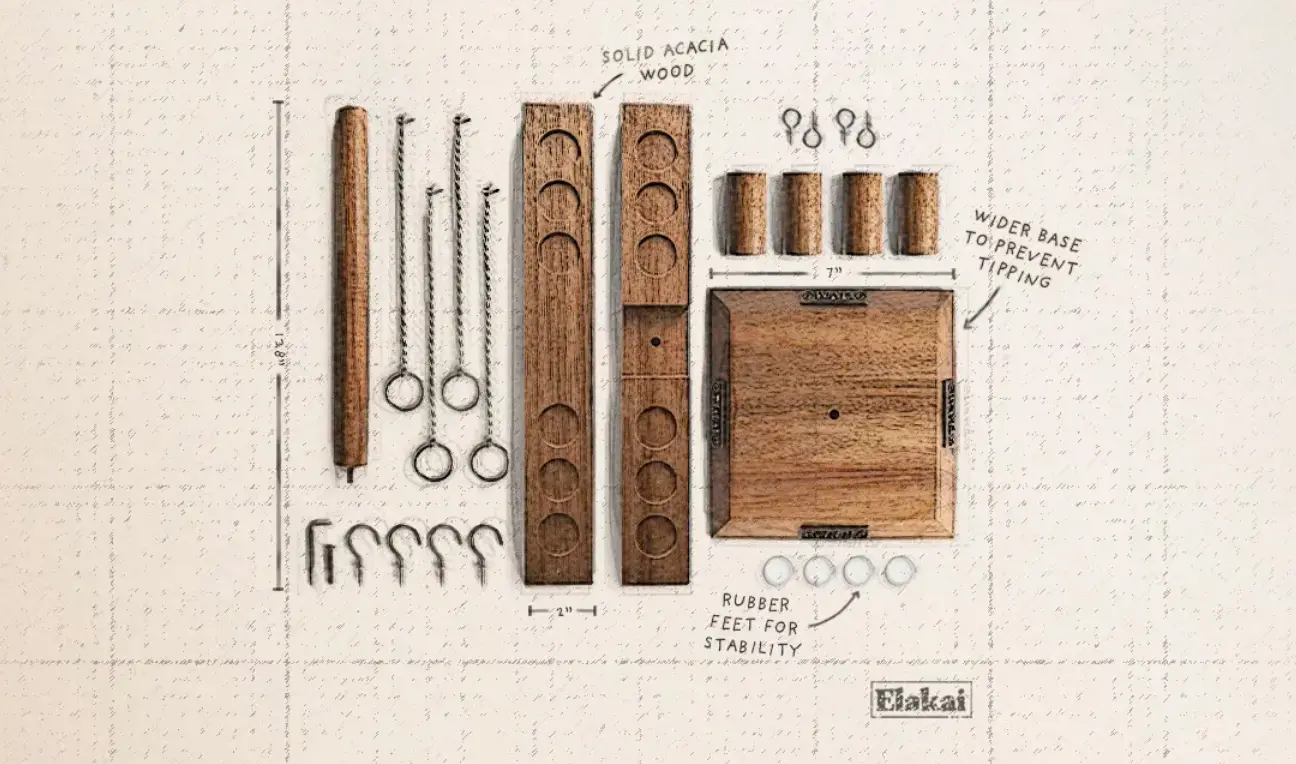











































































































































































































































































































































































































































































































































































































































































































































































































































































































































0