Table of Contents
FEMS Yeast Research Impact
The FEMS yeast research journal is an international journal that publishes original research and reviews. The journal is indexed by Scopus and meets the referencing and indexing criteria of ISO 4. This standard is used to ensure consistency and uniformity in the naming and referencing of serial publications. The journal covers a variety of topics within the fields of Microbiology, Biotechnology, Applied Microbiology, and Yeast Research.
toxicity of yeast Saccharomyces cerevisiae
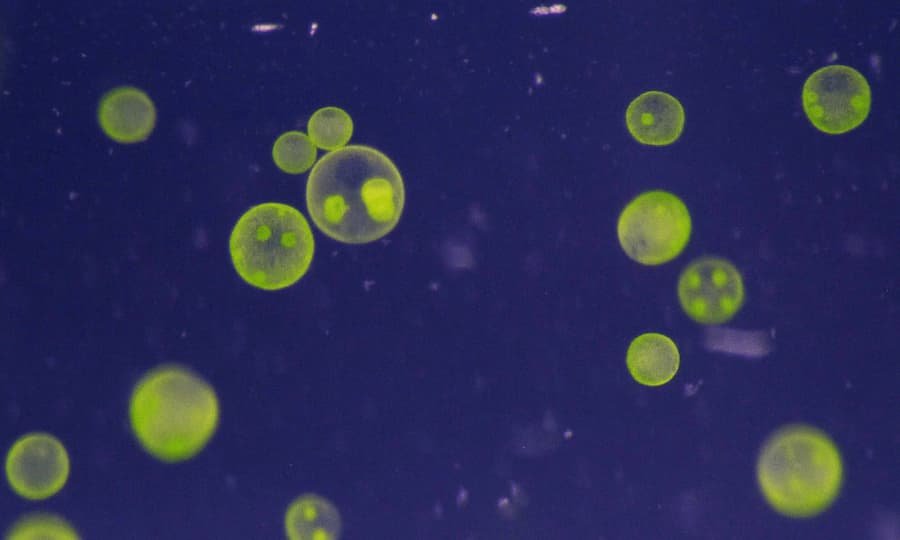
Selenium is a critical trace element for mammals, but it is a toxin in high doses. However, the molecular mechanism for this toxicity remains poorly understood. Because of its high conservation in higher organisms, Saccharomyces cerevisiae has become an excellent model organism for the molecular basis of Se toxicity. In addition, the yeast provides powerful genetic tools for the study of selenium toxicity, such as genome-wide deletion sets and whole-transcriptome and proteome analysis.
Toxins can damage the cell membrane and induce oxidative stress in yeast. Yeast cells respond to oxidative stress by elevating the expression of certain antioxidant genes. This mechanism is important for yeast cells to cope with SDS stress. This pathway is activated when SDS damages the cell wall.
Toxins derived from carbon nanoparticles have been linked to the oxidative stress in yeast. The oxidative stress induced by GN in yeast is known to inhibit the growth of cells and to lead to apoptosis.
Toxins in yeast can cause a variety of diseases. Although it is generally recognized as safe for use in food and hygiene products, recent studies have linked it to skin irritation and oral ulcers. Researchers have also studied the toxicity of synthetic chemicals, heavy metals, and engineered nanomaterials in S. cerevisiae.
High concentrations of GN cause severe physiological changes in yeast, including the induction of early senescence and cell death. These changes may be related to membrane invagination and osmotic stress. They may also play a role in the stability of the fungus.
Several different genes play a role in toxicity in yeast. One gene, YNL134C, plays an important role in detoxifying furfural, while Hsp31p confers protection from reactive oxygen species. Another gene, ECM4, encodes a glutathione transferase located in the cell wall. Moreover, the expression of GRE2 is affected by oxidative stress.
The toxicity of yeast can be mediated by the presence of quinones. These compounds are oxidatively reactive and cause oxidative stress. As a result, they reduce the growth rate of yeast cells.
Effects of cell wall composition on yeast growth
The composition of yeast cell walls is strongly influenced by growth conditions and mode of growth. Changes in the cell wall’s stiffness are dependent on the composition of the b-glucans and chitin, as well as its thickness. Previous studies have found that alterations in yeast cell wall properties can be measured by nanomechanical and biochemical analyses. These changes have been observed during heat, ethanol, and acetic acid stress. But, the exact mechanism is not known.
The cell wall of yeast contains various polysaccharides that are connected in a covalent way. These polysaccharides include a-mannan (soluble in water), alkali-soluble b-glucan, and chitin. In addition, these polymers are bonded together by chitin. The composition of yeast cell walls is a determining factor of yeast health.
Yeast cells were examined by AFM to determine their biophysical properties. They were tested for the Young’s modulus, a quantitative measure of the elasticity of the cell surface. The Young’s modulus was not significantly different in the first 5 h of exponential growth, but the value increased significantly during the induced growth latency. After 7 h, the cells were significantly stiffer than unstressed cells.
These experiments revealed that the cell wall composition of yeast affects its susceptibility to acetic acid stress. The composition of yeast cell wall was influenced by the expression of various genes involved in cell wall synthesis. This study provides a global picture of the time-course of yeast adaptation to acetic acid stress.
The cell wall composition of yeast cells differed with carbon sources. In yeast cells grown on glucose, the cell wall was less sensitive to zymolyse action, while maltose and galactose cultures were more sensitive. Yeast cells grown in ethanol exhibited weaker cell walls than those of yeast grown on glucose.
Increased mannoprotein content was also observed after the application of glycerol to yeast cultures. These changes in cell wall composition would render yeast biomass more efficient as a source of biologically active polysaccharides.
Effects of CO2 on yeast metabolism
Yeast metabolism and growth are affected by carbon dioxide in a variety of ways. This gas has been shown to inhibit the growth of yeast by inhibiting its ability to produce acetyl-CoA. It also inhibits the formation of esters. The effect of CO2 on yeast metabolism has been studied in metabolic flux studies. One such study indicated that the effects of CO2 on yeast metabolism were negligible below a CO2 concentration of 20%.
The effects of CO2 on yeast metabolism are not yet understood, although different sugar chains produce different amounts of carbon dioxide. The results from this study showed that carbon dioxide production increased as the length of a sugar polymer chain increased. However, the levels of carbon dioxide produced by starch were lower than those of other sugar chains. Nonetheless, carbon dioxide production by yeast is a significant limiting factor for the unique organoleptic properties of sparkling wines.
The % of molasses was another significant variable. When the yeast was suspended in 50% molasses, it produced nearly no CO2. This results in reduced CO2 production by the yeast. The results were more significant with three trials than with one. As a result, it is better to use 3 trials than a single trial.
As previously mentioned, carbon dioxide is crucial for yeast metabolism. It makes ATP and glucose available to the yeast cells. Carbon dioxide makes it possible to use these sugars and create ethanol. Both ethanol and ATP are energy-rich compounds for yeast to grow. However, carbon dioxide is also released as a waste product.
Another factor that affects the activity of yeast and its fermentation process is temperature. Temperature can affect the amount of carbon dioxide produced by yeast, which increases the fermentation process. Temperature and the type of sugar used can affect the amount of carbon dioxide produced. Hence, temperature should be controlled carefully.
Peer review process
FEMS Yeast Research publishes a series of peer-reviewed articles and reviews. This publication is edited by Jens Nielsen, a professor at the Chalmers University of Technology in Sweden. He has won several awards for his scientific work, including the Royal Swedish Academy of Engineering Sciences Gold Medal and the ENI Award for Energy Frontiers. He has also written an editorial on the future of scientific publishing.

































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































































0